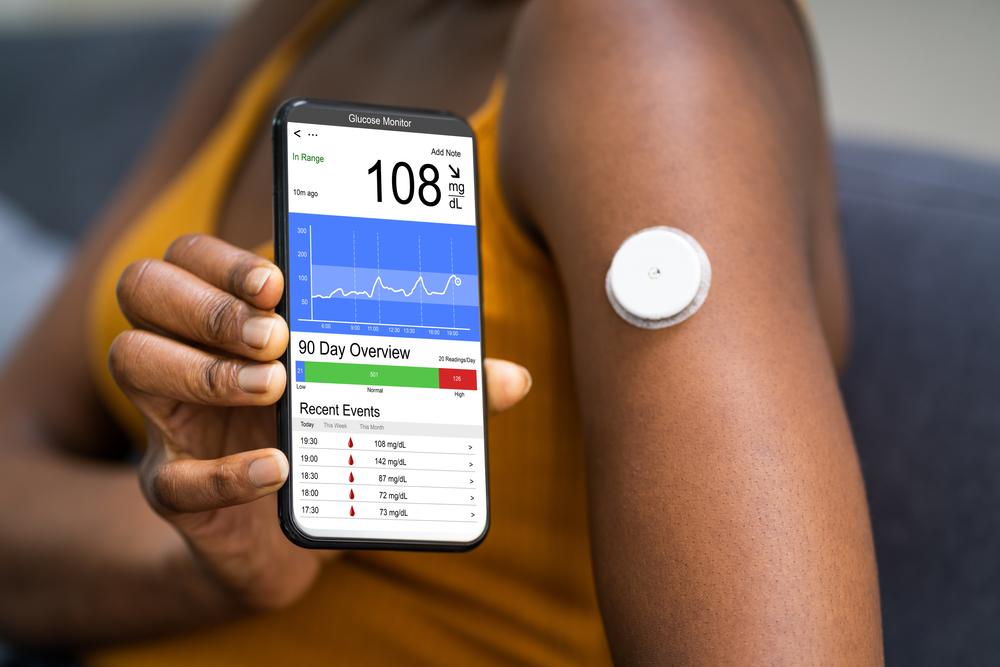
In an unprecedented move, the U.S. Food and Drug Administration (FDA) has approved Dexcom’s continuous glucose monitors (CGMs) for individuals who do not require insulin treatment. This groundbreaking decision marks a significant shift in the management and monitoring of health, broadening the scope of who can benefit from advanced glucose tracking technologies. Traditionally reserved for diabetic patients, Dexcom’s CGMs can now be utilized by a wider audience, keen on gaining deeper insights into their metabolic health and well-being.
What are Continuous Glucose Monitors?
Continuous glucose monitors (CGMs) are state-of-the-art devices that provide real-time readings of glucose levels through a small sensor attached to the body. Unlike traditional glucose meters that require finger-prick blood samples, CGMs offer continuous monitoring, allowing users to track glucose fluctuations throughout the day and night. This constant stream of information can be instrumental in understanding the body’s response to various foods, exercises, and other lifestyle factors, facilitating more informed health decisions.
Dexcom
Dexcom, a leader in diabetes care and management technology, has been at the forefront of developing and commercializing CGMs. Their devices are renowned for their accuracy, ease of use, and the ability to integrate with smartphones and other digital tools, providing users with accessible and comprehensive data about their glucose levels. With the FDA’s recent approval, Dexcom is set to revolutionize how we approach health monitoring beyond the realm of diabetes.
Why You Should Care
This approval is significant for several reasons. It underscores a growing recognition of the importance of personalized health monitoring tools in preventing and managing chronic conditions. By making CGMs available to those not suffering from diabetes, the FDA is acknowledging the role of proactive health management in curbing the rise of metabolic diseases and enhancing overall well-being.
Health Implications
Tracking blood glucose levels can have profound implications for individuals without diabetes. It enables a more nuanced understanding of how lifestyle choices affect metabolic health, potentially unveiling hidden glucose intolerance or insulin sensitivity issues. For those aiming to optimize their diet, improve energy levels, or manage weight, such insight can be invaluable. It empowers users to make data-driven adjustments to their daily routines, fostering a proactive approach to health and longevity.
Takeaway
The FDA’s decision to approve Dexcom’s glucose monitors for non-diabetic use is a monumental step forward in the quest for personalized healthcare. It not only expands the tools available for individual health optimization but also highlights the evolving landscape of health technology. As we continue to embrace these advancements, the potential for improved health outcomes and enhanced quality of life has never been greater. This is a pivotal moment for anyone interested in taking control of their health, marking the beginning of a new era in preventive care and personal wellness.
References
- FDA Clears First Over-the-Counter Continuous Glucose Monitor. Food and Drug Administration website. March 05, 2024. Accessed March 20, 2024. https://www.fda.gov/news-events/press-announcements/fda-clears-first-over-counter-continuous-glucose-monitor