Longevity Drugs Are Already Here — Here’s Why That Matters
This isn’t hype. Since the early 2000s, scientists have largely converged on the key biological drivers of aging — and how to slow them down.
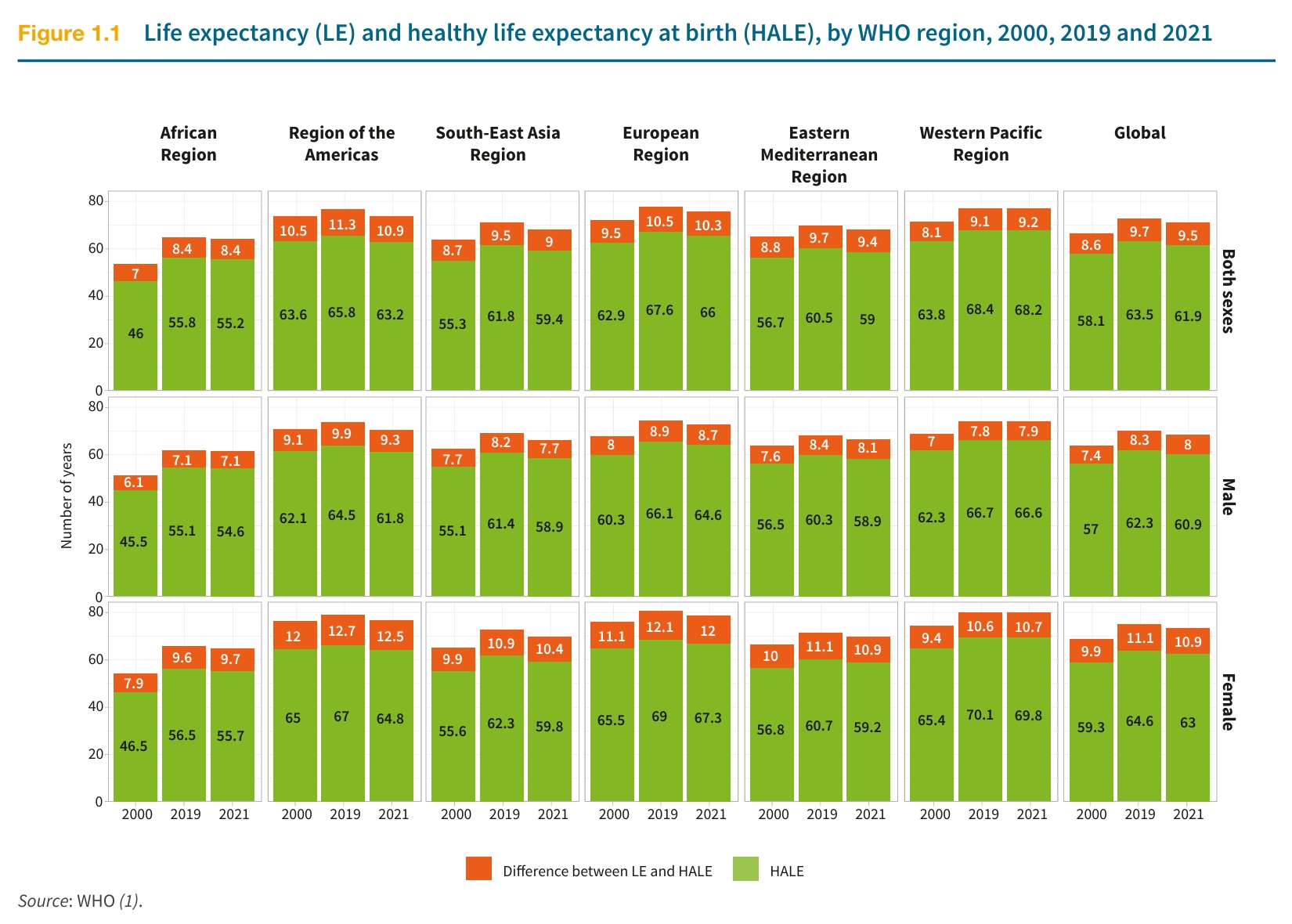
Want proof? Just look at the World Health Statistics Report 2025 from the WHO. Over the past two decades, global life expectancy has risen by 6.5 years. But that steady progress took a hit in 2019, when the COVID-19 pandemic reversed gains, pulling average life expectancy back to where it was 13 years ago.
Global Trends in Life Expectancy and the Limits of Longevity
The WHO’s data on life expectancy (LE) and healthy life expectancy (HALE) from 2000, 2019, and 2021, broken down by region and globally, reveals an important insight: life expectancy is closely tied to a nation’s wealth and healthcare quality. As medical systems advance, population-wide life expectancy tends to rise sharply. But this progress raises a deeper question:
Is there an upper limit to human lifespan?
According to a study published in Nature Aging by S. Jay Olshansky and colleagues at the University of Illinois at Chicago in October 2024, the answer appears to be yes. The researchers found that from 1990 to 2019, the rate of global life expectancy growth has been steadily slowing, and the odds of reaching 100 years of age remain far lower than many assume [1].
This leads to a more personal question:
Would you rather spend 30 years bedridden, or enjoy 20 years of robust health?

Few would choose a longer life confined to a hospital bed over fewer years spent in good health.
That’s why quality of life in old age has become a pressing issue for modern society. In 2001, the World Health Organization introduced the concept of healthy life expectancy (HALE) to highlight this distinction. Many experts now believe that increasing HALE will be the defining goal of what some are calling the second longevity revolution.
During this year’s Two Sessions, renowned host Bai Yansong echoed this view, emphasizing that instead of focusing solely on lifespan, we should prioritize healthspan—the years lived in good health.
But how do we achieve this?
The first step is understanding why we age.
Since the early 21st century, longevity science has made rapid progress, and researchers now share a broad consensus on the biological mechanisms of aging [2].
In 2013, López-Otín et al. introduced the “Nine Hallmarks of Aging” in Cell. A decade later, in 2023, the list was updated to twelve hallmarks: genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, impaired autophagy, dysregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, altered intercellular communication, chronic inflammation, and dysbiosis.
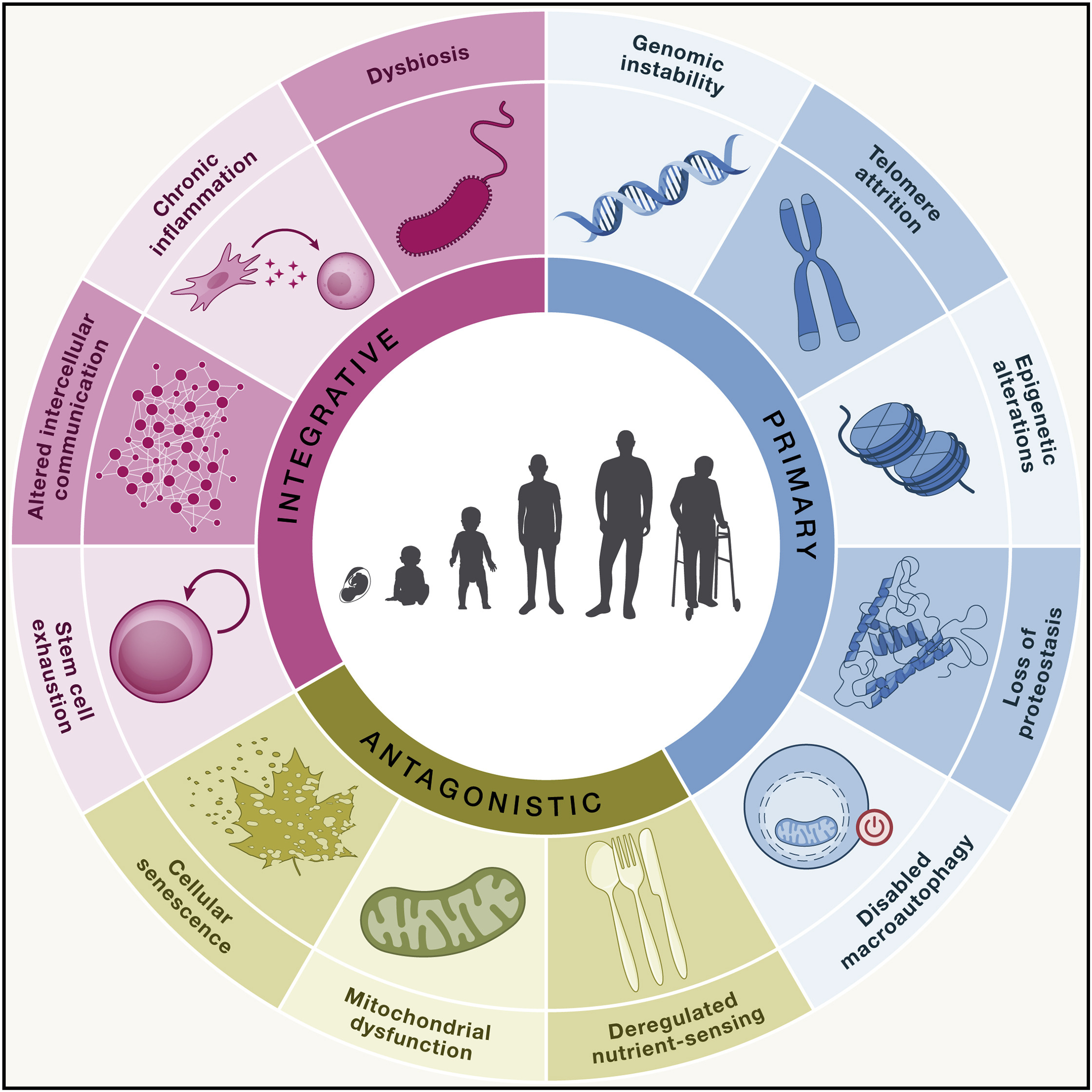
Current interventions in aging science are designed to target one or more of the twelve established hallmarks of aging. Some therapies are highly specific, while others act broadly across multiple aging pathways.
What kinds of interventions are possible?
In aging research, one of the central questions is how to delay aging. But before diving into specific strategies, it’s important to define what we mean by “delaying aging.”
Broadly speaking, there are two main approaches to extending lifespan:
- Reducing all-cause mortality by lowering the incidence of disease or risky behavior. In this sense, quitting smoking and avoiding reckless driving serve similar purposes: both reduce your likelihood of premature death.
- Targeting the biological processes of aging directly. This second approach is our focus—because for people who already enjoy good healthcare and practice healthy lifestyles, the next frontier is breaking through the body’s intrinsic physiological limits.
While risk reduction (Plan 1) remains essential, it is the biological intervention model (Plan 2) that holds the greatest potential for transformative longevity gains.
Mainstream and Well-Studied Aging Interventions
Caloric Restriction
The most researched intervention is caloric restriction (CR)—the deliberate reduction of daily caloric intake without malnutrition. CR mimics a physiological state of energy conservation and has consistently been shown to extend lifespan across species.
Nearly every year, new studies validate its effects in various animal models. One of the most notable in recent years was conducted by Calico, a Google subsidiary, in 2024. Involving nearly 1,000 mice, the study showed that those under the most restricted diets lived nearly 40% longer than those fed ad libitum [3].
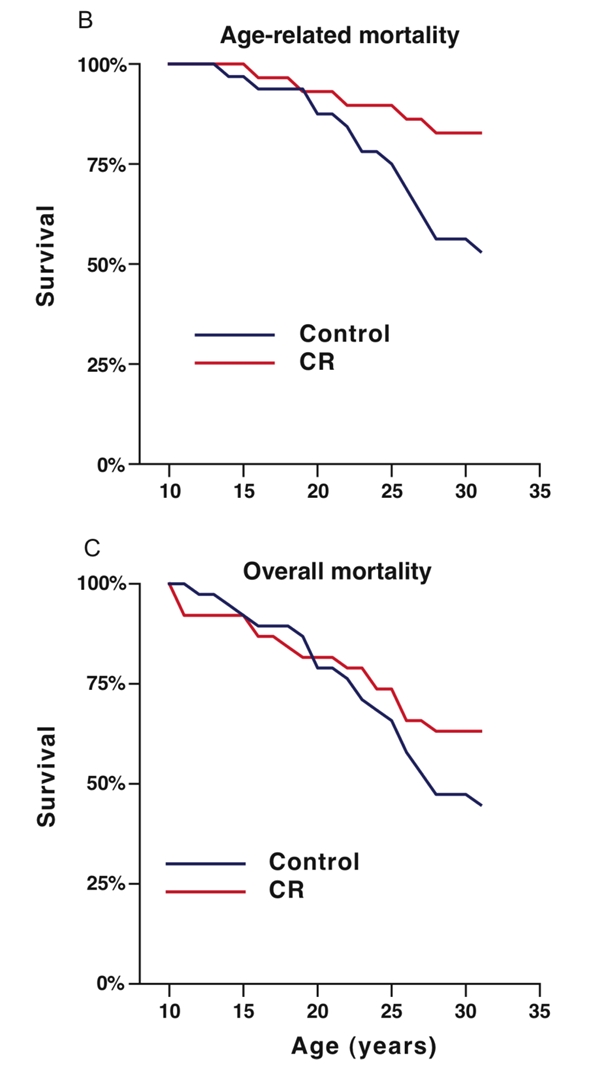
Beyond rodents, the most iconic CR study in primates was jointly conducted by the National Institute on Aging (NIA) and the University of Wisconsin–Madison (UW) beginning in the 1980s. This 30-year, multimillion-dollar trial examined the effects of caloric restriction on rhesus monkeys.
In the UW study [4], monkeys on a calorie-restricted diet not only lived longer but also showed visibly improved health in later life. The differences were so stark they could be observed visually—CR monkeys exhibited fewer signs of aging in appearance and behavior (see panels C and D).
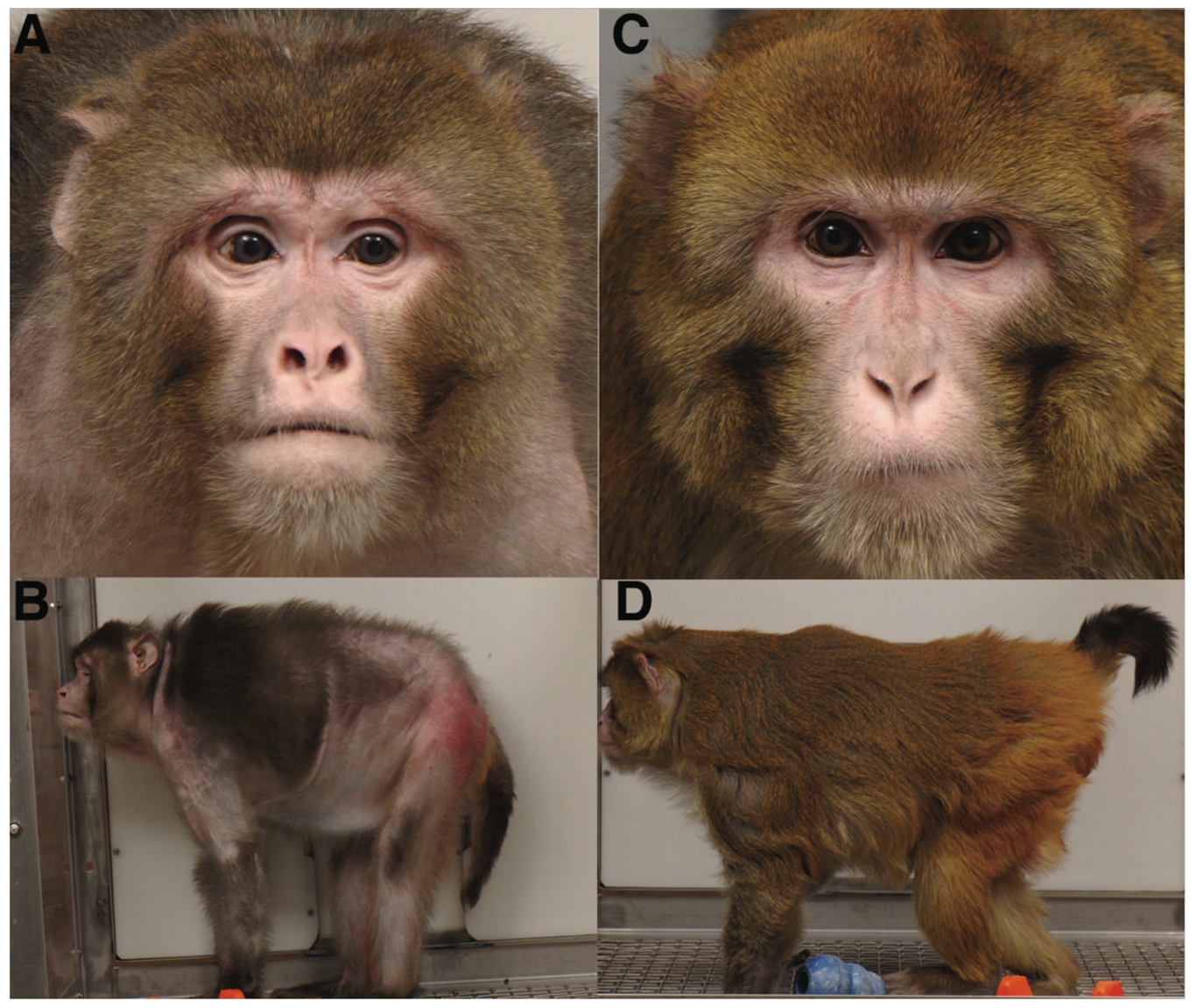
This long-term study provided strong evidence that calorie restriction can positively influence multiple hallmarks of aging, ultimately contributing to extended lifespan.
In humans, fasting protocols such as the “16:8” intermittent fast and the “5:2” diet have been widely studied [5]. These approaches have shown promising results, improving markers like body weight, metabolic function, and muscle performance in various clinical populations.
The mTOR Pathway: A Metabolic Switch Behind the Scenes
In many animal studies, the degree of calorie restriction typically hovers around 40%—a level that is extremely difficult to maintain for most humans. And for many, sacrificing quality of life in pursuit of longevity may not be worth the trade-off.
This led researchers to focus on a key molecular target implicated in the benefits of calorie restriction: the mTOR pathway.
mTOR, or the mammalian target of rapamycin, plays a central role in regulating cell growth and metabolism. It acts like a cellular control chip—monitoring nutrient availability and determining whether a cell should grow, enter rest, or initiate autophagy (self-cleaning) [6].

When cells are exposed to excess nutrients, the mTOR pathway becomes activated—triggering cellular growth and suppressing autophagy.
In contrast, when nutrient levels are low, mTOR signaling is suppressed. This occurs through the phosphorylation of AMPK (AMP-activated protein kinase), which inhibits the activity of mTORC1 (one of the two major mTOR complexes). As a result, cells halt growth and initiate autophagy, the process of cellular cleanup and recycling.
As early as 2001, researchers discovered that inhibiting mTOR could extend lifespan in non-vertebrate species. Over the past two decades, similar effects have been observed in a wide range of organisms—including yeast, nematodes, fruit flies, mice, and even primates—showing that mTOR inhibition can robustly increase both lifespan and healthspan [7].
Calorie restriction itself exerts its life-extending effects largely by suppressing mTOR activity. This insight led scientists to ask a key question:
Can we mimic the metabolic state of calorie restriction—without actual caloric reduction—by selectively inhibiting components of the mTOR pathway?
This idea sparked interest in molecules capable of “tricking” the body into sensing an energy deficit. The first and most well-known of these is rapamycin.
While rapamycin has shown promise in longevity research, it was originally developed as an immunosuppressant and can cause adverse effects in healthy individuals. These include diarrhea, impaired kidney function, respiratory issues, and reduced insulin sensitivity.

These side effects are far from ideal. Even Bryan Johnson—a well-known longevity advocate and early self-experimenter—discontinued rapamycin after experiencing adverse reactions.
In response, researchers have explored alternative approaches. One strategy involves testing everolimus, an isomer of sirolimus (rapamycin’s generic name), which may offer a better safety profile. Others have focused on identifying natural compounds that don’t directly inhibit mTORC1 but instead act through adjacent pathways to indirectly suppress mTOR activity—potentially achieving similar longevity benefits without the downsides.
Glucose Metabolism and AMPK Agonists
Fasting and mTOR inhibition are deeply intertwined with metabolic regulation—pathways that overlap with many of the 12 Hallmarks of Aging.
This has led scientists to revisit established metabolic drugs, with one of the most promising being metformin. Widely used for decades to manage type 2 diabetes, metformin is now being re-evaluated for its potential anti-aging properties.
In 2024, a research team led by Liu Guanghui at the Chinese Academy of Sciences completed a 1,200-day intervention study in crab-eating macaques. Their findings showed that metformin-treated monkeys exhibited multiple markers of slowed aging. Notably, metformin reduced the monkeys’ biological brain age by approximately 6 years [8].
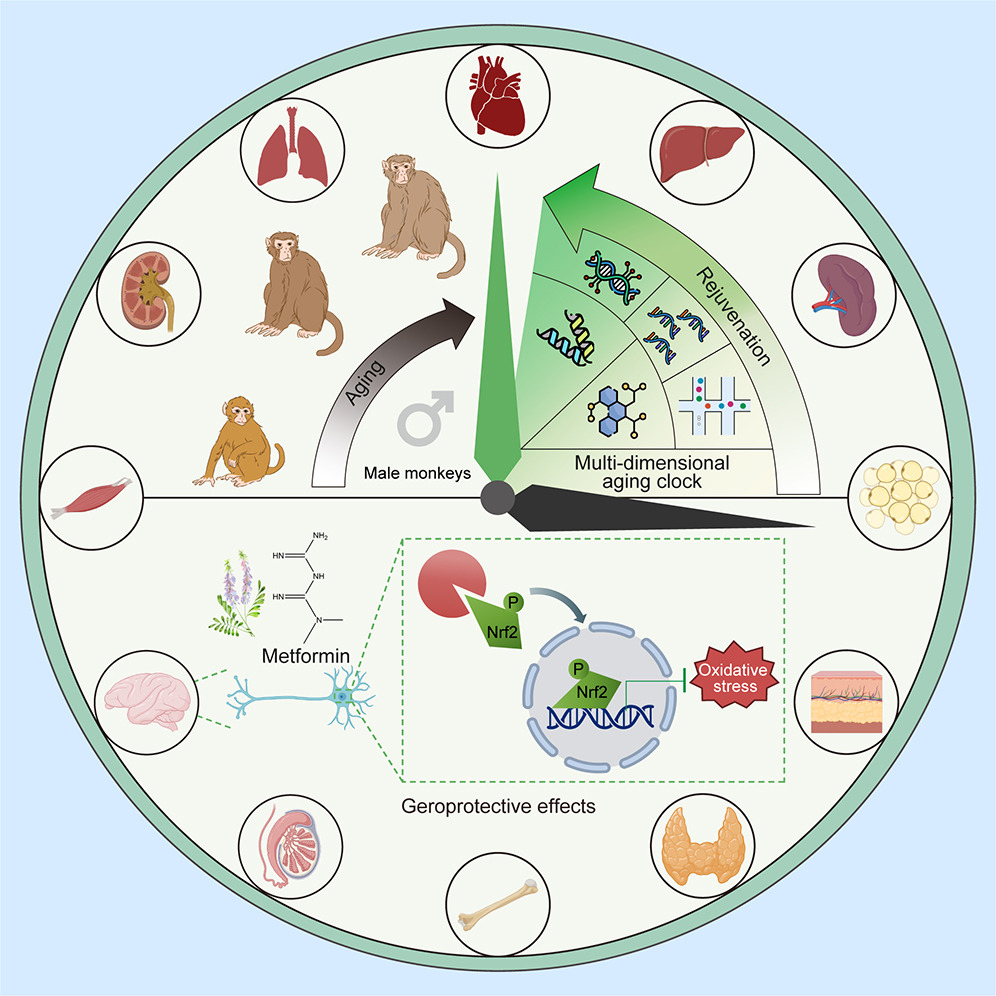
Metformin: Cellular Energy Balance and Longevity
Unlike most other glucose-lowering drugs, metformin’s mechanism of action is uniquely tied to cellular energy balance. Rather than simply controlling blood sugar, metformin reprograms how cells utilize energy—an effect with far-reaching consequences for cellular survival and aging.
Metformin activates AMP-activated protein kinase (AMPK), a central energy sensor in cells. AMPK activation influences a wide range of biological processes, including cell growth, autophagy, and inflammation, all of which are closely linked to age-related diseases such as cancer and neurodegeneration.
Beyond primates, metformin has been tested extensively in mice. In the National Institute on Aging’s Interventions Testing Program (ITP), the combination of metformin and rapamycin significantly extended both median and maximum lifespan across male and female mice—highlighting a robust and reproducible anti-aging effect.
Looking ahead, clinical trials will be critical in validating metformin’s role in human aging. One landmark effort is the TAME (Targeting Aging with Metformin) trial, which plans to enroll 3,000 participants over six years. If successful, TAME could provide the pivotal data needed for metformin to gain official recognition as an anti-aging therapy—potentially delivering better health and longer lives to millions.
NAD+ and the Proteins of Longevity
At the core of aging is metabolism—the way cells produce and manage energy. This concept also ties back to evolution. As evolutionary biologist Richard Dawkins explained in The Selfish Gene, the primary purpose of biological organisms is gene propagation.
In his view, our bodies serve as vessels for genetic transmission. When reproduction is no longer possible, evolutionary pressures weaken—leading to a natural decline in maintenance mechanisms. This is particularly evident in women during menopause, after which signs of biological aging often accelerate. Many of us have seen firsthand how rapidly aging can progress in our mothers following menopause.

Is There a Master Regulator of Aging?
Could there be a single molecular target that drives aging across multiple systems—one that, if restored, might reset the clock? Dr. David Sinclair of Harvard Medical School believes so.
Sinclair has focused on NAD⁺ (nicotinamide adenine dinucleotide), also known as coenzyme I. NAD⁺ is not a newly discovered molecule; it’s long been recognized as an essential coenzyme in cellular metabolism. Coenzymes act as helpers that assist enzymes in carrying out biochemical reactions.
Put simply, NAD⁺ is like a critical “fuel additive” for the cell—enabling the conversion of nutrients into ATP, the energy currency of life. Without NAD⁺, this process cannot proceed efficiently.
In a landmark 2013 study published in Cell, Sinclair’s team showed that NAD⁺ levels decline significantly with age, leading to mitochondrial dysfunction and contributing to systemic aging [9]. The findings suggested that restoring NAD⁺ could help preserve mitochondrial health and slow down the aging process at multiple biological levels.
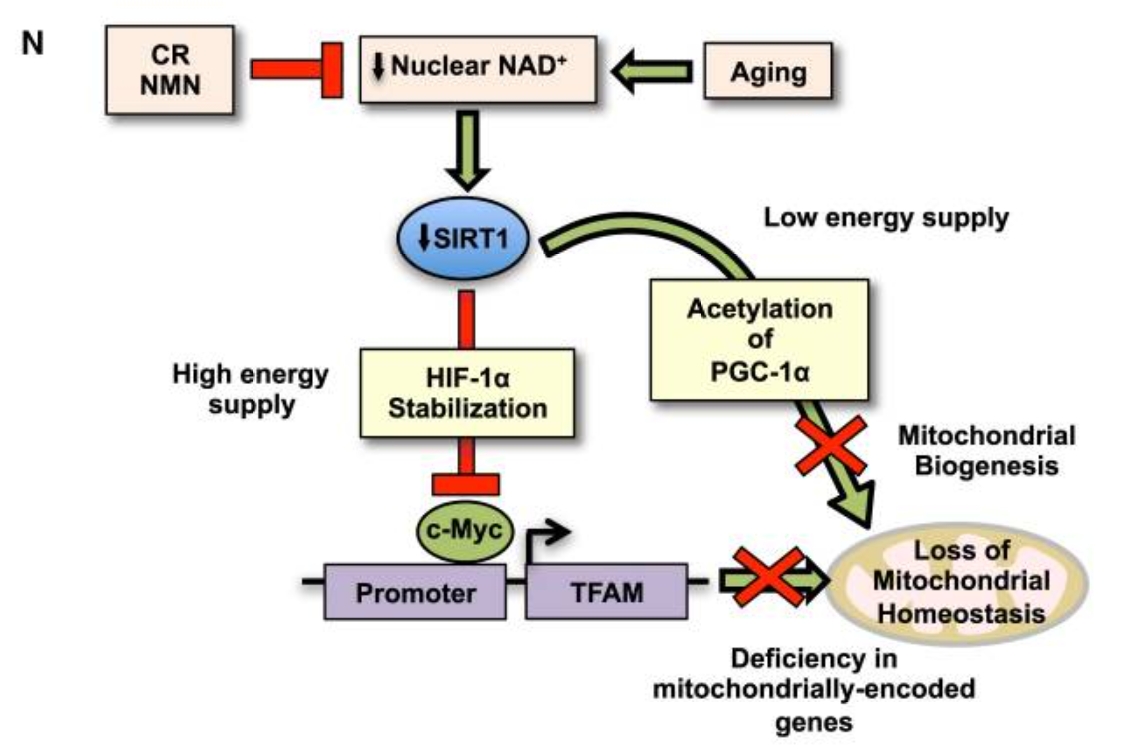
Restoring NAD⁺: A Potential Key to Longevity
Follow-up studies by Sinclair and other researchers revealed that increasing NAD⁺ levels could enhance mitochondrial function and improve skeletal muscle health in aged mice—ultimately leading to lifespan extension [10].
Given its broad benefits, one might ask: Can we simply supplement NAD⁺ directly?
While NAD⁺ is a naturally occurring and relatively safe molecule—especially compared to drugs like rapamycin or even metformin—it poses a challenge: the molecule is too large to be efficiently absorbed through the digestive tract when taken orally.
To address this, Sinclair’s team identified two NAD⁺ precursors, NMN (nicotinamide mononucleotide) and NR (nicotinamide riboside). These molecules are part of the body’s natural NAD⁺ biosynthesis pathway. Through a series of experiments, researchers found that supplementing with NMN or NR could restore NAD⁺ to youthful levels and reactivate NAD⁺-dependent enzymes like Sirtuins and CD38 [11].
As a result, NAD⁺ research has expanded rapidly. Clinical studies now suggest that boosting NAD⁺ may offer multiple benefits: delaying skeletal muscle aging, enhancing endurance, improving glucose metabolism, increasing insulin sensitivity and cardiovascular function, restoring muscle strength, and even enhancing memory and learning capacity [12].
Sirtuins: The Longevity Proteins
Within the NAD⁺ metabolic pathway, a critical group of enzymes comes into focus: the Sirtuin family, often referred to as “longevity proteins” or SIRT proteins. These enzymes function as deacetylases, regulating cellular processes like gene expression, metabolism, and stress resistance. Studies across various species have shown that organisms with more active Sirtuins tend to live longer.
Why? Sinclair and others have proposed that Sirtuins are closely linked to epigenetics—a system that governs how genes are expressed without altering the DNA sequence. To use an analogy: genes are like recipes, while epigenetics are the chefs. During aging, the recipes remain the same, but the chefs grow sloppy, and the resulting “dishes”—our cellular functions—decline in quality.
Chinese Academy of Sciences researchers Liu Guanghui, Qu Jing, and Zhang Weiqi have contributed significantly to this area, showing that Sirtuins regulate energy metabolism and play a major role in slowing the aging process [13].
To enhance Sirtuin activity, scientists have explored both NAD⁺ supplementation and direct activators like resveratrol and ginsenosides—natural compounds shown to stimulate SIRT function [14].
Despite evolving strategies, NAD⁺ remains at the heart of these interventions. Since 2025, even newly spotlighted molecules like ergothioneine have been found to exert anti-aging effects via NAD⁺ pathways.
Whether NAD⁺ is the “ultimate antidote” to aging remains to be seen, but no other molecule currently comes closer to addressing aging at its metabolic and epigenetic core.
Senolytics: Clearing Out the Aged
There’s another, more direct idea in aging research: if senescent cells are harmful, why not eliminate them?
This is the concept of Senolytics, first introduced by James Kirkland at the Mayo Clinic. Kirkland’s team discovered that aging cells often enter a senescent state and begin secreting a mix of harmful factors known as the Senescence-Associated Secretory Phenotype (SASP). These factors fuel chronic inflammation and are linked to conditions like insulin resistance and tissue dysfunction [15].

Kirkland’s team later discovered that a natural compound called quercetin, when combined with the cancer drug dasatinib, could effectively eliminate senescent cells in aged animals. This combination—known as D+Q—not only cleared senescent cells but also reversed certain aging-related conditions and restored youthful function in experimental animals [16]. Substances capable of selectively clearing senescent cells were named Senolytics.
Today, numerous research groups are testing alternative senolytic compounds. Promising candidates include fisetin, a plant-derived flavonoid, and BCL-2 inhibitors, which disrupt survival pathways in senescent cells. These emerging molecules may offer more targeted and effective senolytic therapies in the future.
Why Do Most Longevity Breakthroughs Stop at Animal Studies?
This question might seem complex, but the answer is simple: humans live a long time.
As mentioned earlier, global average life expectancy has surpassed 70 years. Because so many variables affect human lifespan—from genetics to environment to behavior—identifying a single compound that can reliably extend life in people is an enormous challenge.
To address this, scientists first turn to model organisms like mice. Mice have short lifespans, are genetically well-characterized, and are highly responsive to experimental interventions—making them indispensable tools in aging research.
Why Most Longevity Research Stalls Before Reaching Humans
The largest and most authoritative screening program for anti-aging compounds is the Interventions Testing Program (ITP), launched in 2002 by the National Institute on Aging (NIA). Its goal is to evaluate the effects of various compounds on lifespan by testing them in mice under standardized conditions.
Unlike many studies that use genetically modified or disease-prone strains, the ITP uses wild-type mice—offering a more realistic model for general aging. These mice have a median lifespan of 700–900 days, meaning it takes approximately 5.5 years to obtain final lifespan data from a single study.
This timespan alone presents logistical challenges. A PhD program typically lasts 5–6 years—about the same time it takes to complete one full round of ITP testing. The timelines are even more daunting for primate models. The calorie restriction study on rhesus monkeys by NIA and UW took 30 years, while Liu Guanghui’s metformin study in cynomolgus monkeys required 12 years.
Moreover, primate studies are exponentially more expensive than mouse studies. A single monkey can cost hundreds of times more than a mouse—and that’s assuming perfect compliance from the animal subjects.
When it comes to human studies, the complexity increases dramatically.
In 2025, Harvard nutrition expert Hu Bingchang published a 30-year observational study tracking dietary patterns in over 100,000 individuals [17]. While the dataset is valuable, it relied on Food Frequency Questionnaires (FFQs) administered every two years—asking participants to recall their dietary habits over the prior 24 months. This method, though common in nutritional epidemiology, is difficult to replicate in randomized controlled trials aimed at testing aging interventions.
Even with unlimited funding and personnel, such trials face significant ethical hurdles. Designing a long-term, double-blind study to delay aging in healthy adults is not only impractical—it’s unlikely to pass ethical review boards. Common sense alone reveals the challenges of executing a decades-long placebo-controlled trial on aging.
As a result, most aging intervention research remains at the preclinical stage, with human trials primarily focused on safety and disease-specific outcomes [18].
Is Aging a Disease?
This leads to a fundamental debate: Should aging itself be treated as a disease?
Dr. David Sinclair argues that it should be. In nearly every talk and interview, he repeats a bold claim: “Aging is a disease—and a treatable one.” The Wall Street Journal called this a “seductive notion,” though critics argue it could be socially harmful.
Labeling aging as a disease raises complex ethical and societal questions. If every elderly person is considered a “patient,” does that imply fragility or dependency? Could this framing unintentionally reinforce ageism or deny older adults their agency, success, and dignity?
On the other hand, treating aging as a disease has practical benefits. It would allow older adults to recognize physical decline not as inevitable, but as a biologically modifiable condition. It could also unlock medical and policy support, directing funding and regulatory frameworks toward aging interventions.
The World Health Organization briefly classified aging as a disease in ICD-10, before downgrading it in ICD-11. While China has not yet approved any pharmaceuticals specifically for aging, it continues to support healthy aging through public health initiatives. The government’s “Healthy China Action (2019–2030)” specifically identifies extending healthy life expectancy as a national priority.
The Commercialization of Longevity Science
Despite the regulatory and ethical hurdles, aging science has rapidly commercialized, attracting interest from elite scientists, entrepreneurs, and billionaires.
Aging waits for no one—and the business world has noticed.
Harvard’s David Sinclair, for example, has founded or advised dozens of companies, including Elysium, which markets consumer NAD+ supplements, and Senolytic Therapeutics, focused on clearing senescent cells. His extensive involvement in industry has been covered in-depth by The New York Times and The Wall Street Journal—underscoring the commercial viability of longevity science.
Other institutions are following suit. The Mayo Clinic, where James Kirkland conducts his senescence research, has invested in multiple biotech startups, further cementing its strategic position in the aging biotech ecosystem.

Big Tech and Global Capital Are Betting on Longevity
Beyond research institutions and hospitals, the world’s wealthiest companies and individuals have been investing heavily in aging interventions for years. Notable examples include:
- Calico, backed by Google
- Altos Labs, funded by Amazon founder Jeff Bezos
- Retro Bioscience, supported by OpenAI co-founder Sam Altman
These organizations have successfully recruited leading scientists and are beginning to generate both scientific discoveries and translational breakthroughs in aging biology.
This trend isn’t limited to international markets. In China, domestic institutions—including state-owned enterprises—are also entering the longevity space. According to Xinhua News Agency, Sinopharm Group formed a joint venture with U.S.-based pharmaceutical innovator Seragon in 2023, launching Sinopharm Seragon. With support from Harvard University and the Mayo Clinic, the company is advancing commercialization of SRN-901, an experimental therapy for aging-related conditions.
Regardless of opinion, the pace of development is accelerating.
Why Longevity Matters
Of course, we all want to live longer—but more importantly, we want to live well in our later years.
As emphasized by Bai Yansong during China’s “Two Sessions” political meetings, the focus should shift from simply extending lifespan to maximizing healthy life expectancy—the number of years a person can live in good health and functional independence.
That is the true promise of aging interventions: not just more time, but more time in health, strength, and dignity.

The Broader Impact of Extending Healthspan
The societal value of extending lifespan—particularly healthy lifespan—is profound. A U.S. study using a micro-simulation model compared two scenarios: delaying aging versus targeting individual major diseases. The results were striking: delaying aging increased average life expectancy by 2.2 years, with most of that added time spent in good health.
Beyond personal well-being, the study estimated this shift could generate $7.1 trillion in economic value over the next 50 years. It would also significantly reduce the burden of chronic disease and disability in the elderly population [19].
A parallel analysis by the Institute of Finance and Economics at the Chinese Academy of Social Sciences reached a similar conclusion. The report emphasized that closing the gap between healthy life expectancy and average life expectancy would foster the accumulation of high-quality human capital, stimulate technological innovation, and support high-quality economic growth [20].
In short, extending healthy lifespan is not just a personal benefit—it is a national and global strategic priority with far-reaching economic and societal implications.

Longevity Shouldn’t—and Won’t—Be Just for the Wealthy
Given the growing momentum in longevity science, there’s no need to fear that “longevity drugs” will become exclusive tools of the ultra-rich. Like all transformative technologies, aging interventions will follow a familiar path: initially expensive and limited, then gradually democratized through innovation, competition, and policy support.
Consider the case of aspirin. When Bayer launched it in 1899, a single tablet cost the modern equivalent of a few dollars. Today, thanks to technological advancement and widespread adoption, aspirin is available to virtually everyone—for just a few cents per dose.
The same economic trajectory applies to aging intervention therapies. While early-stage treatments may carry high costs due to research intensity and limited production, these prices will inevitably fall as science matures, markets expand, and access broadens.
In short, making longevity accessible to all isn’t just an aspiration—it’s an achievable outcome rooted in the very structure of scientific and economic progress.
References:
- Olshansky, SJ, Willcox, BJ, et al. Implausibility of radical life extension in humans in the twenty-first century. Nat Aging. 2024 Nov;4(11):1635-1642.
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: An expanding universe. Cell. 2023;186(2):243-278.
- Di Francesco A, Deighan AG, Litichevskiy L, et al. Dietary restriction impacts health and lifespan of genetically diverse mice. Nature. 2024;634(8034):684-692.
- Colman RJ, Anderson RM, Johnson SC, et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325(5937):201-204.
- Zhao D, Guallar E, Woolf TB, et al. Association of eating and sleeping intervals with weight change over time: The Daily24 cohort. Journal of the American Heart Association. 2023;12(3).
- Papadopoli D, Boulay K, Kazak L, et al. mTOR as a central regulator of lifespan and aging. F1000Research. 2019;8:998.
- Mannick JB, Lamming DW. Targeting the biology of aging with mTOR inhibitors. Nature Aging. 2023;3(6):642-660.
- Yang Y, Lu X, Liu N, et al. Metformin decelerates aging clock in male monkeys. Cell. 2024;187(22):6358-6378.e29.
- Gomes AP, Price NL, Ling AJY, et al. Declining NAD+ Induces a Pseudohypoxic State Disrupting Nuclear-Mitochondrial Communication during Aging. Cell. 2013;155(7):1624-1638.
- Kane AE, Chellappa K, Schultz MB, et al. Long-term NMN treatment increases lifespan and healthspan in mice in a sex dependent manner. bioRxiv (Cold Spring Harbor Laboratory). June 2024.
- Poljsak B. NAMPT-Mediated NAD Biosynthesis as the internal timing mechanism: In NAD+ world, time is running in its own way. Rejuvenation Research. 2017;21(3):210-224.
- Alegre GFS, Pastore GM. NAD+ precursors nicotinamide mononucleotide (NMN) and nicotinamide riboside (NR): Potential dietary contribution to health. Current Nutrition Reports. 2023;12(3):445-464.
- Bi S, Jiang X, Ji Q, et al. The sirtuin-associated human senescence program converges on the activation of placenta-specific gene PAPPA. Developmental Cell. 2024;59(8):991-1009.e12.
- Pearson KJ, Baur JA, Lewis KN, et al. Resveratrol Delays Age-Related Deterioration and Mimics Transcriptional Aspects of Dietary Restriction without Extending Life Span. Cell Metabolism. 2008;8(2):157-168.
- Palmer AK, Kirkland JL. Aging and adipose tissue: potential interventions for diabetes and regenerative medicine. Experimental Gerontology. 2016;86:97-105.
- Xu M, Pirtskhalava T, Farr JN, et al. Senolytics improve physical function and increase lifespan in old age. Nature Medicine. 2018;24(8):1246-1256.
- Tessier AJ, Wang F, Korat AA, et al. Optimal dietary patterns for healthy aging. Nature. March 2025.
- Harinath G, Lee V, Nyquist A, et al. Safety and efficacy of rapamycin on healthspan metrics after one year: PEARL Trial Results. medRxiv (Cold Spring Harbor Laboratory). August 2024.
- Goldman DP, Cutler D, Rowe JW, et al. Substantial health and economic returns from delayed aging may warrant a new focus for medical research. Health Affairs. 2013;32(10):1698-1705.
- Zhang Yingxi, Xia Jiechang. How does the improvement of healthy life expectancy promote economic growth? ———An empirical study based on cross-national macroeconomic data. Management World. 2020, 36(10): 41-53+214-215.












