Picture tiny power plants nestled inside every cell of your body—mitochondria. These microscopic structures tirelessly produce energy, ensuring your cells function smoothly. But just like any factory, mitochondria aren’t invincible. With age, they begin to break down, becoming less efficient, leaking harmful molecules, and even damaging surrounding cellular structures. This process, termed mitochondrial dysfunction, is a cornerstone of aging science, deeply influencing how our bodies age. And just like any other power plant that falls into disrepair, the process of mitophagy is you body’s way to recycling these power plants into new, more functional ones.
When Good Power Plants Go Bad
As mitochondria age, they become leaky, releasing reactive oxygen species (ROS). Think of ROS as sparks flying from faulty wiring, capable of igniting a harmful blaze within cells. ROS can damage DNA, proteins, and fats, contributing to chronic inflammation—a phenomenon known as inflammaging. This persistent inflammation silently accelerates the aging process and fuels numerous age-related diseases.
Understanding Mitophagy
Fortunately, our cells have developed an elegant cleanup mechanism known as mitophagy. Mitophagy acts like an internal recycling center, recognizing damaged mitochondria, breaking them down, and recycling their components. This critical process ensures only healthy mitochondria remain operational, preserving cellular health.
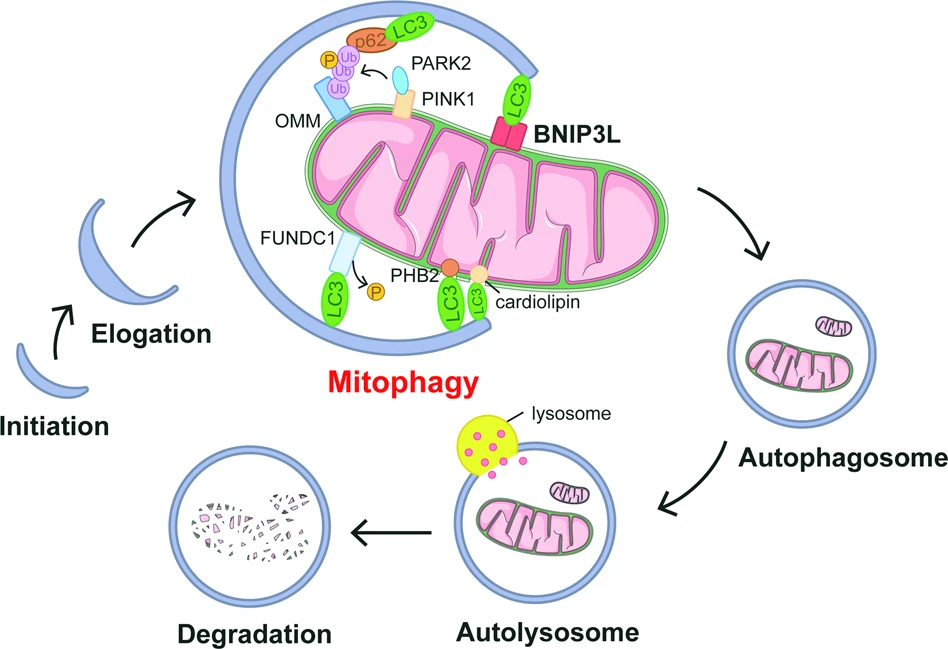
Mitophagy is primarily managed by a couple of key pathways. The best-known route involves proteins called PINK1 and Parkin, which tag faulty mitochondria for destruction. Alternative pathways involve other markers such as BNIP3, FUNDC1, and NIX, particularly when cells experience stress or low oxygen conditions.
Why Mitophagy Falters
Mitophagy operates as a vital cellular quality-control system, clearing away damaged mitochondria to preserve energy efficiency and limit oxidative stress. In younger cells, this system is highly responsive and dynamic. But as we age, the regulation and execution of mitophagy become impaired. Studies of aging skeletal muscle reveal a decline in mitophagy-related proteins such as PINK1 and Parkin, along with diminished lysosomal capacity. This inefficiency contributes to an accumulation of dysfunctional mitochondria, leading to compromised cellular respiration, reduced ATP production, and increased production of reactive oxygen species (ROS). These changes directly correlate with sarcopenia, fatigue, and metabolic disorders frequently observed in older adults.
In the brain, mitophagy is equally crucial. Neurons, which are highly reliant on mitochondrial energy, show signs of mitochondrial stress and accumulation with age. The aging brain attempts to compensate by increasing mitochondrial turnover, but this compensation comes at a cost. Elevated turnover without efficient clearance can lead to fragmented mitochondria, incomplete mitophagy cycles, and buildup of toxic mitochondrial debris. This dysfunction has been linked to neurodegenerative conditions such as Alzheimer’s and Parkinson’s diseases, where impaired mitophagy is now considered a key contributor to disease progression.
Adding complexity, age-related changes in nutrient sensing, NAD+ availability, and the unfolded protein response in mitochondria (UPRmt) also reduce the cell’s ability to properly initiate mitophagy. These upstream signals are essential for detecting damage and triggering the cleanup response. As these signaling pathways degrade with age, the very systems meant to preserve mitochondrial health become sluggish or misdirected. The result is a vicious cycle—damaged mitochondria fuel chronic inflammation (inflammaging), which in turn further suppresses mitophagy and accelerates tissue degeneration. This breakdown of intracellular housekeeping marks a pivotal shift in how aging impairs resilience at the cellular level.
Enhancing Mitophagy
Scientists have begun exploring innovative ways to boost mitophagy, providing promising insights into slowing the aging process. Genetic engineering in animal models, particularly mice, has already demonstrated significant benefits. By genetically enhancing mitophagy, researchers observed remarkable decreases in mitochondrial damage, improved metabolism, and even notable lifespan extensions.
Additionally, lifestyle interventions can naturally enhance mitophagy. Regular physical exercise and dietary interventions like intermittent fasting have shown remarkable efficacy in increasing mitochondrial turnover and enhancing cellular cleanup. Even certain supplements, notably melatonin—a hormone often associated with sleep regulation—can stimulate mitophagy. Recent studies highlighted melatonin’s ability to rejuvenate skin health through mitophagy activation.
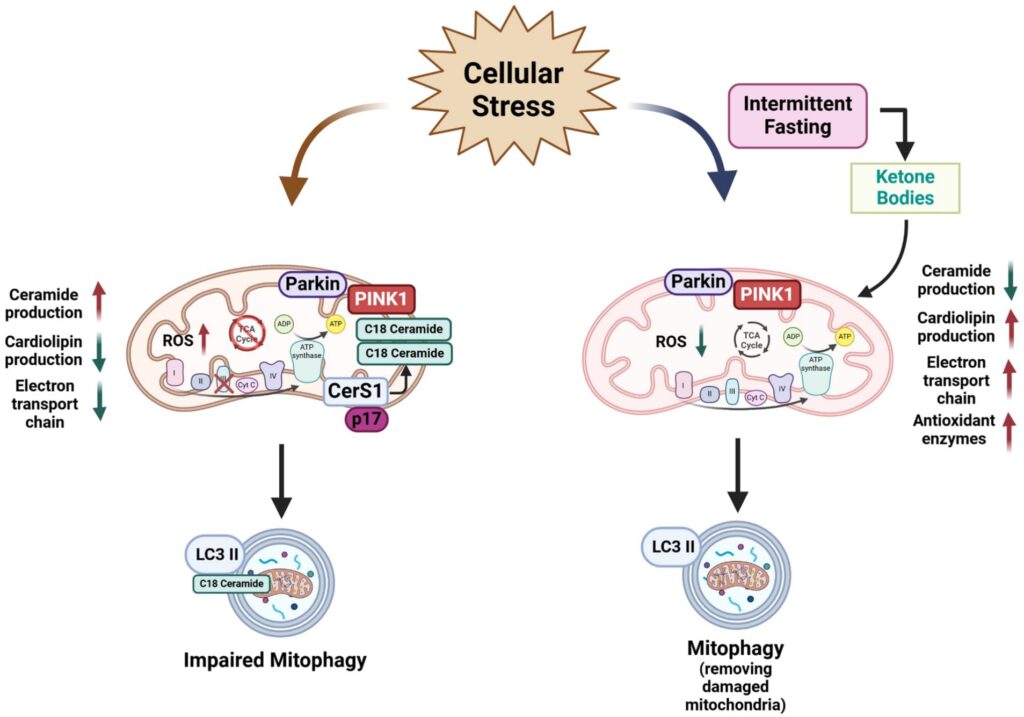
Another critical discovery involves the mitochondrial unfolded protein response (UPRmt), the cell’s internal stress alarm system that maintains mitochondrial quality. When mitochondrial proteins misfold, the UPRmt activates, deploying protective proteins like SIRT3, antioxidant defenses, and enhancing mitophagy. This sophisticated response mechanism, extensively studied from worms to mammals, emphasizes the delicate balance required to maintain mitochondrial and overall cellular health.
Complex I Modulators as Therapeutic Innovators
Enter a new frontier with compounds like leramistat and MBS2133—novel Complex I inhibitors recently explored in groundbreaking research. Unlike harsh mitochondrial poisons, these modulators finely tune mitochondrial function. They decrease harmful ROS production while simultaneously activating cellular stress responses beneficial to cellular health.
By introducing mild stress (known scientifically as hormesis), these Complex I modulators push mitochondria to shift from mere energy producers to active repair and regeneration hubs. Clinical studies in models of rheumatoid arthritis and pulmonary fibrosis have already demonstrated their therapeutic potential, markedly reducing inflammation, preventing fibrosis, and stimulating tissue repair.
The Future of Mitochondrial Health
Imagine combining lifestyle changes like regular exercise and balanced diets with carefully selected supplements and pharmaceuticals to optimize mitochondrial function and mitophagy. The future of aging intervention might involve comprehensive treatment plans integrating NAD+ boosters, mitophagy enhancers, and precise mitochondrial modulators.
Such a combination strategy could fundamentally change our relationship with aging, turning it from a process of inevitable decline into one of sustained health and vitality. With ongoing research, compounds that gently modulate mitochondrial activity and robustly support mitophagy could become standard in our toolkit for promoting healthier, longer lives.
The Grand Story of Mitochondrial Health
Think of your body as a dynamic ecosystem, continuously striving for balance and renewal. Mitochondria and mitophagy are central characters in this epic narrative, dictating the rhythm of aging and influencing the quality of our lives. Just as communities thrive when they maintain their infrastructure, cells prosper when mitochondria function efficiently and waste management (mitophagy) remains effective.
Understanding and enhancing mitochondrial health through targeted interventions provides us with profound opportunities—not just to live longer but to live better. The science of mitochondria and mitophagy isn’t merely academic curiosity; it’s a gateway to transforming the human aging experience.
Embrace this knowledge and consider every healthy choice as a proactive step towards maintaining your cellular ecosystem. The journey of aging is ongoing, but armed with the power of mitochondrial health, we can reshape its narrative toward vitality, resilience, and rejuvenation.
So which is better? Mitophagy vs. Complex I Inhibitors
While both mitophagy activators and Complex I inhibitors aim to restore mitochondrial integrity and enhance tissue health, they operate through distinct biological mechanisms—and each offers unique strengths. Mitophagy activators enhance the cell’s natural recycling system, promoting long-term mitochondrial quality control without disrupting core bioenergetic functions. In contrast, Complex I inhibitors induce a hormetic stress response, redirecting cell fate programs toward regeneration and inflammation resolution, especially under pathological conditions like fibrosis or rheumatoid arthritis.
| Feature | Mitophagy Activators | Complex I Inhibitors |
| Primary Action | Enhance mitochondrial cleanup | Induce hormetic stress for repair |
| Mechanism | Activates PINK1/Parkin, UPRmt, etc. | Mildly inhibits ETC to modulate signaling |
| Mildly inhibits ETC to modulate signaling | Preventive, long-term mitochondrial health | Therapeutic, targeting disease pathology |
| Notable Agents | Melatonin, NAD+ boosters, Spermidine | Leramistat, MBS2133 |
| Effect on Inflammation | Indirect reduction via cleanup | Direct suppression and resolution |
| Risk of Overuse | Low (natural modulation) | Moderate (requires dosing precision) |
| Best for | Maintenance, longevity, healthspan | Regeneration, fibrosis, immune resolution |
When comparing their utility, mitophagy activators may be better suited for ongoing mitochondrial maintenance and prevention, while Complex I inhibitors could offer more targeted, therapeutic benefits in disease reversal or acute tissue remodeling. A synergistic strategy, combining both approaches, may ultimately deliver the most comprehensive benefits for healthy aging and chronic disease management.
Sources
- Phenotypic Pharmacology of Novel Complex I Inhibitors Eliciting Tissue Repair in Chronic Disease — As reported in Journal of Pharmacology and Experimental Therapeutics, 2025; detailing effects of leramistat and MBS2133 on Complex I PubMed
- Pickles S, Vigie P, Youle RJ. Mitophagy and Quality Control Mechanisms in Mitochondrial Maintenance. Current Biology. 2018;28(4):R170–R185. Nature
- Relevant review: Molecular mechanisms and physiological functions of mitophagy, EMBO Journal 2020; emphasizes Pickles et al.’s pathway overview. ScienceDirect
- Wang Y, et al. Melatonin regulates mitochondrial dynamics and mitophagy via AMPK signaling. Journal of Pineal Research. 2024. AMPK-PINK1 activation improving mitochondrial health Nature
- Lopez‑Otín C, et al. Hallmarks of Aging: An Expanding Universe, Cell. 2023;186(1):21–45. Cell
- Zhang H, Ryu D, et al. NAD+ repletion improves mitochondrial and stem cell function and enhances lifespan in mice. Science. 2016;352(6292):1436–1443. ScienceDirect
- Fang EF, et al. NAD+ augmentation restores mitophagy and limits accelerated aging in Werner syndrome. Nature Communications. 2019;10(1):5284. Nature
- Zhang Q, Wu X, et al. The mitochondrial unfolded protein response is mediated cell‑non‑autonomously by retromer‑dependent Wnt signaling. Cell. 2018;174(4):870–883. Berkeley Library
- Palikaras K, Lionaki E, Tavernarakis N. Coordination of mitophagy and mitochondrial biogenesis during ageing in C. elegans. Nature. 2015;521(7553):525–528. PubMed
- Ni HM, et al. BNIP3‑ and FUNDC1‑Mediated Mitophagy Protects against Mitochondrial Dysfunction in Hypoxic Conditions. Autophagy. 2012;8(10):1657–1670. (PubMed confirmed existence.) PubMed